Explain the Difference Between the Two Kinds of Substances
Here we will study both categories. In contrast mixtures contain two or more substances so they can be separated.

Homogeneous Vs Heterogeneous Mixtures Difference And Comparison Diffen
Evaporation takes place at the surface of a liquid such as a puddle drying up.

. By their chemical composition pure substances get divided into two types elements and compounds. A Elements and compounds are two kinds of substances. A Elements and compounds are two kinds of substances.
As was the case for gaseous substances the kinetic molecular theory may be used to explain the behavior of solids and liquids. 1 Pure substance The substances that contain only one type of particle and they are free from any mixture are known as pure substances. Ontology is the branch of philosophy that studies concepts such as existence being becoming and realityIt includes the questions of how entities are grouped into basic categories and which of these entities exist on the most fundamental level.
Atoms of the same element are identical in properties. Substances which have a specific composition and cannot be separated into any constituents are called pure substances. Heterogeneous and homogeneous mixtures.
We can distinguish between the different substances by observing physical properties of these substances. The pure substances possess similar properties and composition throughout on the other hand in mixtures properties and composition vary as the constituents are mixed in indefinite proportion. The combination of two or more pure substances is called a mixture.
Pure substance cannot be separated into two or more substances by any mechanical or physical method. Are pure substances made up of only l type of atom. The main difference between simple diffusion and facilitated diffusion is that simple diffusion is an unassisted type of diffusion in which a particle moves from higher to a lower concentration across a membrane whereas facilitated diffusion is the transport of substances across a biological membrane through a concentration gradient by means of a.
Physically a person with an addiction to any substance will suffer withdrawal symptoms if they suddenly stop. If there are no phase change between two or more substances the substance that there is the most of is considered the solvent while all the rest are solutes. Mixtures show the properties of the pure substances in it.
The boiling point of a substance is proportional to the strength of its. A polar bond is formed by the attraction between oppositely-charged ions. Adhesion is the attractive force between two types of molecules which are different from each other and cohesion is the intermolecular force between two similar molecules.
A pure substance is made up of the same kind of molecules whereas mixture is made up of two different molecules. Once abuse has turned into an addiction they no longer can control the effects of the addiction. An intermolecular force is an attractive force that arises between the positive components or protons of one molecule and the negative components or electrons of another molecule.
You can predict an ionic bond will form when two atoms have different electronegativity values and detect an. Gold silver iron and aluminium are pure substances to name a few. When a person is struggling with substance abuse they still have control over their life.
Various physical and chemical properties of a substance are dependent on this force. In PubChem terminology a substance is a chemical sample description provided by a single source and a compound is a normalized chemical structure representation found in one or more contributed substances. Three types of chemical substances are solidsliquids and gases.
Ontology is sometimes referred to as the science of being and belongs to the major branch of philosophy known as metaphysics. For example sodium and chloride form an ionic bond to make NaCl or table salt. Therefore the properties are uniform throughout the sample.
The answer of this question depends on which two substances are chosen to differentiate. Atoms that participate in an ionic bond have different electronegativity values from each other. A suspension is a mixture where components must be forced to distribute evenly.
Therefore the key difference between adhesion and cohesion is that the adhesion is the attraction between substances or molecules which are not similar whereas cohesion is the. Pure substances are further divided into elements and compounds. Physical mixtures is a physical combination of matter in an proportion.
Boiling takes place below the surface of a liquid and causes bubbling like a pot of water on a hot. On the other hand when it comes to explaining what theyre trying to explain in this case something like the nature of consciousness Substance dualism gets to just say this is easy the mental is just a whole nother fucking thing thats the explanation while property dualism has to be all What if the way consciousness comes from the brain is RESPECTED. Passive mediated transport is the movement of molecule with the concentraion gradient but a membrane transport protein helps the molecule cross by either a protein channel or a carrier protein.
View the full answer. Researchers concluded that there are similarities and differences between diagnostic symptoms of drug addiction and behavioral addiction. Pure substances are a chemical combination of matter in definite proportions.
Behavioral addictions such as gambling overeating television compulsion and internet addiction are similar to drug addiction except that the individual is not addicted to a substance but heshe is. They need the substance just to feel normal. The periodic table contains a list of the only elements currently known to man.
Note that we will use the popular phrase intermolecular attraction to refer to attractive forces between the particles of a substance regardless of whether these. The two types of vaporization are evaporation and boiling. Explain the differences between them using copper sulfur and copper I sulfide.
If any of the substances changes phase while making the solution it is considered a solute. Mixtures can be classified into two types viz. B How many electrons protons and neutrons are in a neutral atom of the following isotope of krypton8436 Kr.
Solids are rigid and have fixed shape with definite volume whi. There are two types of pure substances. In diffusion the molecules are moving from greater concentration to lesser concentration with the concentration gradient.
The question is not surprising as the names substance and compound alone do not inherently convey the difference. In the following description the term particle will be used to refer to an atom molecule or ion. If you look at the difference between cotton and wool cotton is softer and easier to clean than wool.
Pure substances are homogenous. Elements cannot be broken down into anything simpler physically or chemically. For example salt water is a physical mixture and distilled water is a pure substance.

Relationships Between The Types Of Matter And The Methods Used To Separate Mixtures Matter Worksheets Matter Science Chemistry

Classification Of Matter Ck 12 Foundation

Compound Vs Mixture Difference And Comparison Diffen

Re Post Element Compound And Mixture Tyas Physics Compounds And Mixtures Elements Compounds And Mixtures School Science Experiments

What Are The Types Of Pure Substances Compounds Elements Videos

Pure Substances And Mixtures Venn Diagram Examples Venn Diagram Worksheet Venn Diagram Template

What Is A Substance Definition Types Examples Video Lesson Transcript Study Com
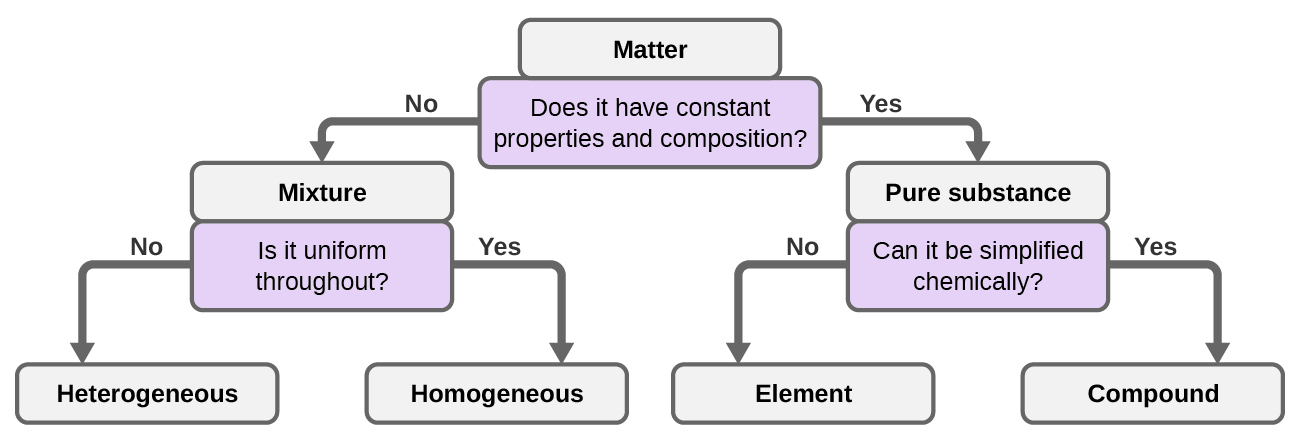
1 2 Phases And Classification Of Matter Chemistry
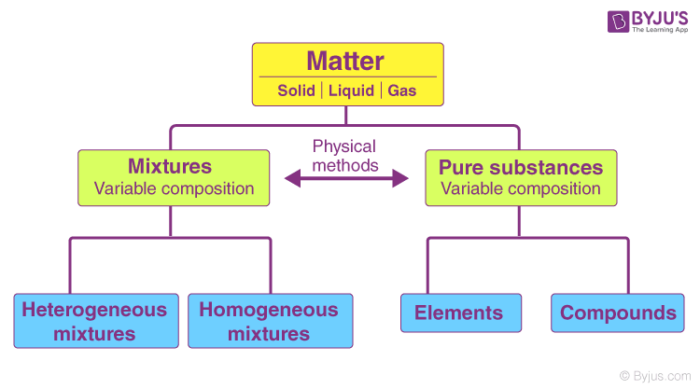
What Is Pure Substance Definition Examples Difference Between Pure Substance Mixture

3 5 Pure Substances And Mixtures Chemistry Libretexts

Classification Of Matter Boundless Chemistry

What Is A Substance Definition Types Examples Video Lesson Transcript Study Com

Classification Of Matter Boundless Chemistry

What Is A Substance Definition Types Examples Video Lesson Transcript Study Com
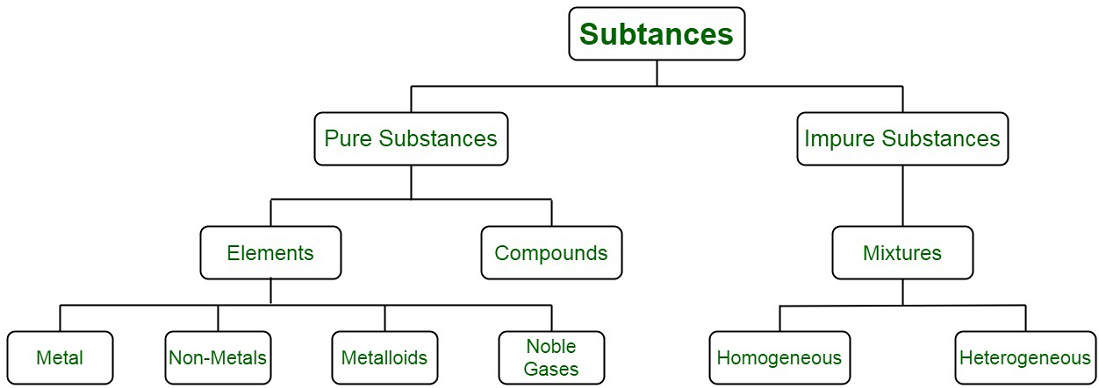
Pure And Impure Substances Geeksforgeeks

What Are The Types Of Pure Substances Compounds Elements Videos

Matter Pure Substances And Mixtures Matter Worksheets Matter Science Chemistry

Pure Substance Or Mixture Matter Science Physical Science Middle School Chemical Science
